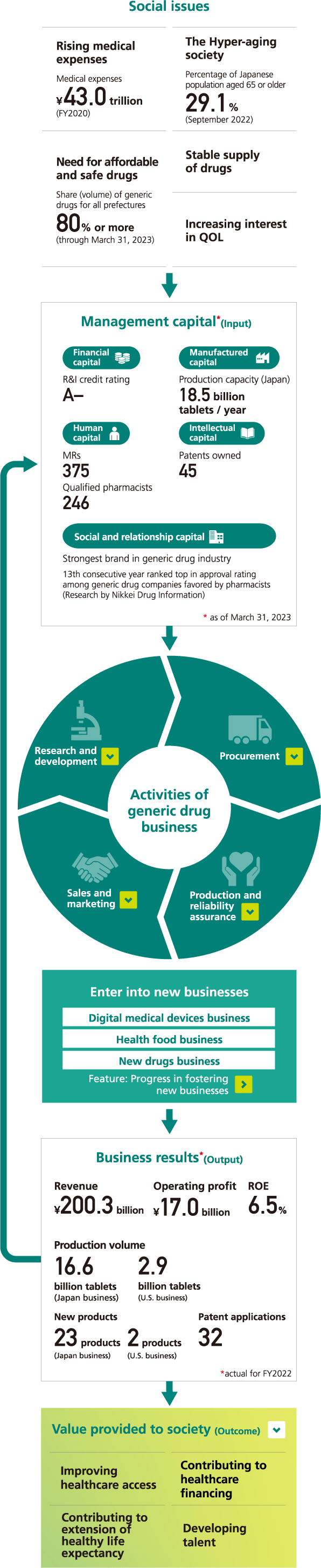
Value creation activities and Sawai’s strengths
Research and development
Strength 1Ability to undertake research and analysis of original drug patents
By utilizing both human assets with extensive experience in patent trials and proceedings and a proprietary database that includes information on patents and proceedings in Japan and overseas, we implement an extremely sophisticated patent strategy. We develop the optimal patent strategy for new issues in collaboration with patent attorneys in Japan and overseas who possess broad knowledge of pharmaceuticals.
In recent years, we have also been keeping an eye on intellectual property trends in the U.S. and Europe and leverage those trends for our intellectual property strategy for Japan. In particular, it is possible to learn a lot from applying for paragraph IV certification in the U.S., and this is a major asset that supports future business activities, which is one of our strengths.
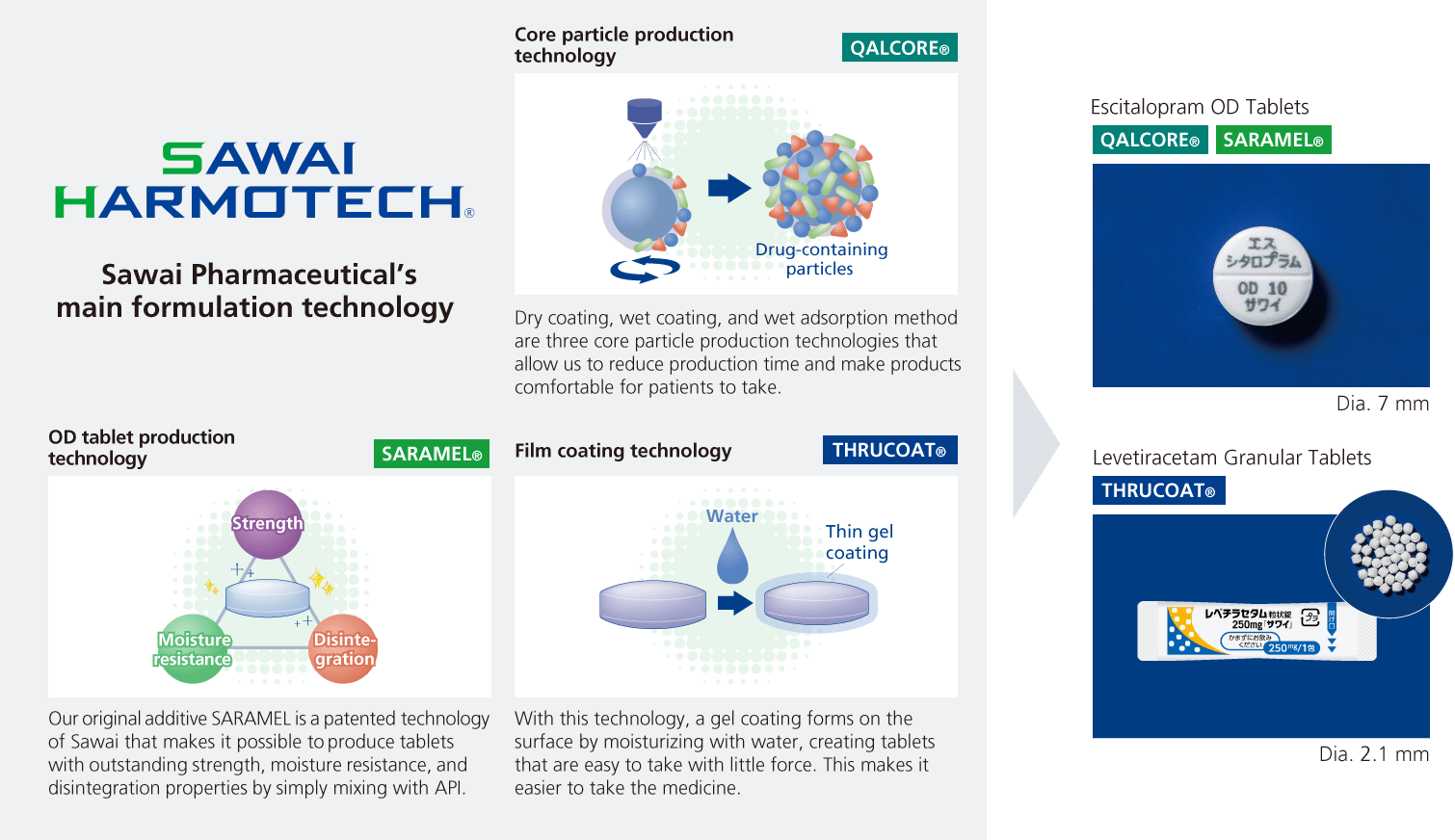
Strength 2Formulation technology capabilities based on human assets with expertise in API properties and formulation technology
Another of our Group’s strengths is our formulation technology capabilities, one aspect of which is collecting the latest information related to APIs and formulation from throughout the world, and this makes it possible to conduct development in line with international harmonization. Furthermore, we have increased the certainty of our product launches by continually revising and bringing forward development plans. Even while busy with product development, we actively take on the challenge of research linked to future development topics and aggressively work to accumulate and expand our formulation technology.
We will provide drugs that meet the needs of healthcare professionals and patients and introduce first-of-their-kind products using SAWAI HARMOTECH®, a series of formulation technologies born from this research.
Procurement
Strength 1Strong new product API research and procurement abilities
As for APIs used in new development, we undertake various activities such as searching for APIs throughout the world, examining production facilities, quality, etc., and conducting analysis and trial production of pharmaceuticals using samples of these APIs. We use APIs that meet our own standards, which are even higher than those of the Ministry of Health, Labour and Welfare. The API Sourcing Group provides support for research and procurement through its specialists in purchasing, including members with experience at new drug manufacturers, API trading companies, and Sawai factories.
Strength 2Selection and procurement of raw materials with primary emphasis on quality and safety
In order to deliver even higher quality generic drugs, we inspect the factories of API manufacturers, particularly the Quality Assurance Department. Furthermore, we confirm that API manufacturers’ quality management systems meet Sawai standards by checking that the Production Department undertakes manufacturing in an appropriate environment and the Quality Control Department conducts appropriate analysis. We also release information on the country and factory where APIs are manufactured to provide healthcare professionals with peace of mind. In addition, we actively undertake multisourcing—that is, procuring the same API from multiple manufacturers in order to ensure stable procurement.
Production and reliability assurance
Strength 1Manufacturing know-how for high-mix, low-volume production
To produce various types of generic drugs using the same machinery, it is vital to possess know-how regarding preventing the mixing of principal ingredients.
For tablet presses that form granules into tablets, it takes almost one whole day to switch between products, and Sawai ensures safety by validating each cleaning procedure. This know-how related to validating unique generic drug production processes and managing production makes it possible to conduct high-mix, low-volume production and ensure quality.
Strength 2Stringent quality management
Quality is managed to exceed government standards through all process, from selection of API and additives to production process, and even post-sales.
We are working to further improve our uncompromising quality in order to provide generic drugs that can be used with peace of mind.
Strength 3Own production factories located throughout Japan and external partner companies
Production management and quality control are undertaken, primarily for our eight main factories in Japan. All eight factories observe GMP, and we strive to implement continual improvements by acting in concert and sharing the findings of audits.
We also conduct audits of formulation manufacturing subcontractors at least once every three years.
Sales and marketing
Strength 1Provision of accurate information
We provide information to patients and healthcare professionals through three channels—approximately 370 medical representatives (MRs); the Medical Information Center, an inquiry desk open 24 hours a day, 365 days a year; and a website.
In addition to undertaking activities for providing accurate information to all healthcare professionals, MRs collect and compile information on side-effects and safety of drugs, and this work is led by the Pharmacovigilance Department. We are working to have our drugs properly used by providing that information to healthcare workplaces as feedback.
Strength 2Extensive product lineup
The lineup of products offered by our Japanese Group companies extends to about 800 products. Our ability not only to collect and provide information on various diseases and in a wide range of fields but also to broadly meet the treatment policies and needs of healthcare professionals, which is possible because we market numerous products, is another strength of Sawai Group.
On account of our continuing training for MRs that covers various products, MRs can acquire extensive knowledge. It is precisely because of our wide lineup of products, that we can propose multiple drugs to treat the same disease and more concomitant drugs.
Value provided
Creating a society in which everyone can receive healthcare with peace of mind
Throughout the world, including Japan, many people lack access to sufficient healthcare for financial reasons.
The Group strives to improve access to healthcare by developing and offering a stable supply of high-quality, high valued-added generic drugs.
Aiming to become a general healthcare company that supports people’s health
In not only its core generic drug business but also a wide range of fields that extend from prevention to treatment,
the Sawai Group provides choices not limited to health foods and drug therapies, including digital and medical devices,
but has also entered the new drug business for rare diseases.
























 Working to establish a production system at Trust Pharmatech
Working to establish a production system at Trust Pharmatech